Tag Archives: covid19
Patent pool as a tool to tackle COVID-19 Pandemic

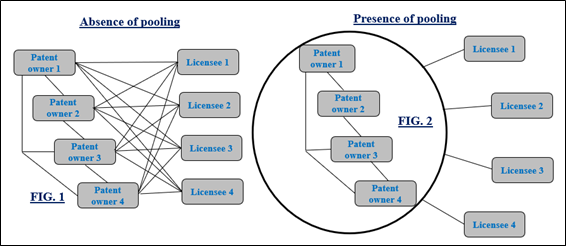
No wonder, innovation steers today’s world. In many instances, several enterprises develop technologies that end up overlapping in various aspects. In scenarios like these, possibilities of one technology to infringe on other technologies is well likely to arise. To address this, patent pools were created. According to WIPO, patent pool is defined as “agreement between multiple patent owners for licensing their patents to one another or any other third party for the purpose of sharing their intellectual property rights.” In the absence of a patent pool (FIG. 1), each of the licensee must negotiate with all the patent owners for obtaining license, which is time consuming and expensive process. Contrastingly, in the presence (FIG. 2), licensees can avail the patent rights as one package from the pool resulting in simplification and cutting down transaction costs.
For a patent pool to work, it requires most key patent holders to be in the pool. As long as members holding the IP positions are participating to ensure that patent pool gains “critical mass” and it is well administered, patent pool will work, otherwise it will fail. The first patent pool was established in mid 1850s and, gained momentum in 2000s when the USPTO encouraged creation of patent pools for biotechnology patents to combat concerns governing biotechnology patents. In the years 2000-2005, discussions on establishing a patent pool began due to SARS outbreak, during which several corporates had filed patent applications on the “genomic sequence” of the virus that was responsible for the outbreak.
Amidst the pandemic COVID-19- Pooling
In 2020, the pandemic COVID-19 has shown benefits of collaborative research in many ways. The TRIPS agreement allows countries to grant compulsory licenses to companies to produce a patented product at times of emergencies. In view of this, the World Health Organization (WHO) and the European Union strongly supported patent pooling, whereas the US and UK were against the patent pooling for pharmaceutical drugs in the 73rd World Health Assembly held virtually on 18-19th May 2020. At the World Health Assembly, Costa Rica suggested pooling of rights to deal with pandemic through free or affordable licensing to ensure the outputs from the efforts could be used by countries with limited economic resources to deal with the issue. This proposal received recognition from all over the globe, except US and UK. Amidst the COVID-19 outbreak, Medicine Patent Pool (MPP) has been actively aiding in removing any IP-related barriers. MPP is a United Nations international organization founded in July 2010, by Unitaid. It is a global health initiative that collaborates with potential partners to make medical innovations to diagnose and treat major diseases in low-and-middle income countries and negotiate agreements with pharmaceutical companies. MPP has been mustering information pertaining to medical patents for COVID-related patents. It seeks voluntary licenses from the patent holders of anti-retroviral drugs to form pooled resource of patented innovations. Through this initiative, pharmaceutical companies and innovators can access the pooled patent rights to develop the new and adapted innovations required for sale in developing countries. The trouble and expense of negotiating licenses where several patent holders might hold rights in a single innovation can be mitigated. Patent holders are benefitted by receiving royalty from various countries and a collaborative platform for easy access to develop the need, as the price of licensing the already patented technology to develop new medicines can be brought down by patent pools.
MPP has created various online databases such as MedsPaL for patents relating to medicines such as remdesivir, tocilizumab and VaxPaL specifically for patents related to COVID-19 vaccines. Another commendable initiative is taken by the WHO, by creation of a voluntary patent pool known as COVID-19 technology access pool (C-TAP), led by Costa Rica and WHO. The goal of C-TAP patent pool is to bundle multiple pieces of IP together to reduce the transaction costs of knowledge sharing on a patent-to-patent basis. MPP is regularly updating its patent intelligence database, MedsPaL, with the status of products during COVID-19 and will continue to update as and when the new patents emerge to find the cure during the COVID-19 pandemic. Currently, Remdesivir, Lopinvir/Ritonavir, Favipiravir, Tocilizumab and Sarilumab have been updated in the database.
Patent pooling: India’s perspective
Patent pooling is recent concept in India. The Indian Patent Act, 1970 neither provides for nor obstruct the creation of patent pools. Nonetheless, one could consider Sections 68, 69 and 102 from Patent Act, 1970 to avail patent pooling with terms and conditions making such relaying legally valid. However, there are issues pertaining to conflicts between the Competition Act, 2002 and Patent Pooling, as the said Act disallows any types of agreements or licenses which have an adverse effect on fair competition in India. With respect to Indian scenario, the Competition Act, 2002 prohibits various IPR licensing agreements that are calculated to be of anti-competitive natured. When such a conundrum arises, Section 140 of the Indian Patent Act defines certain conditions to be considered while getting into a licensing agreement in relation to licensing of a patented article. Hence, it can be contemplated that the Patent Act, 1970 has certain provisions to address the issues coming out of patent pools. The idea of a patent pool particularly may be useful for a generic drug manufacturing major like India. The pool can help with faster production of drugs which are found to be effective for the pandemic. Recently, one Indian generic drug manufacturer Aurobindo Pharma Limited joined the Medicines Patent Pool (MPP) to manufacture several anti-retroviral medicines. This step would facilitate Aurobindo Pharma to have access to the patented drugs of “Gilead Sciences” which was recently introduced into the pool.
Conclusion:
Patent pools can be highly beneficial for facilitating innovation, accelerating the development of a medicine for COVID-19, specially amidst the outbreak. It will be a win-win situation as the patent holders will receive royalties for their innovations, thereby maintaining their income while the low-and middle-income countries would get access to the much-needed medications at affordable prices. For the generic drug manufacturing companies like India, the companies can combine different medications into singe/fixed doses to create better medicines. As in, back in 2014, ViiV Healthcare contributed to MPP by providing dolutegravir, an antiretroviral drug for HIV to its pool resource. It allowed generic drug manufacturing companies to create an affordable version of anti-HIV drug. In India, for the development of commerce, the knowledge regarding the concept of patent pooling should be known to every inventor so as to get good progress in the field of technology or pharmaceuticals. Creation of pandemic patent pool at a global level to pool innovations is good to promote access to medicines and protect public health in large scale.
Please feel free check our services page to find out if we can cater to your requirements. You can also contact us to explore the option of working together.
Best regards – Team InvnTree
This work is licensed under a Creative Commons Attribution-Non Commercial 3.0 Unported License
Members of European Parliament back India-South Africa proposal for suspension of COVID-19 vaccine patents

The Proposal:
In early October, India and South Africa had jointly proposed for a moratorium to the Trade-Related Aspects of Intellectual Property (TRIPS) agreement that protects intellectual property and international trade, vis-a-vis the current pandemic. It was argued that the TRIPS agreement was going to delay an affordable access of COVID–19 related medical products and the R&D related to manufacturing and supply of the COVID–19 related products. The two countries called for a global solidarity for supporting the means to respond to COVID–19 rapidly. They argued that a waiver of the TRIPS treaty for a specific period of time can allow manufacturing of generic versions of the potential vaccine without violating any of the international trade rules by speeding up the collaboration among WTO member-states.
The Debate:
Many developed countries and a few developing countries were of an opinion that a solution can be found within the TRIPS agreement itself without having to waiver few provisions of the TRIPS agreement on COVID related products. They argued based on the following:
- Countries can resort to an alternative way such as compulsory licensing or government use of a patent without authorization of the proprietor.
- IP offices of Individual Countries may opt to undertake fastrack programs to speed up the granting of COVID related IP to facilitate dynamic trade landscape. For instance, the US Patent and Trademark Office (USPTO) recently initiated a Covid-19 Prioritized Examination Pilot Program.
- The opponents of the waiver pointed out that the Article 73 in the TRIPS agreement that refers to the security exceptions for member countries allowing exceptions to be considered for national security purposes. However, some entities have also resorted to “Open COVID pledge” in order to offer free licenses to others, thereby concluding that IP is not a stopper but a catalyst for finding the best solution for the pandemic.
Recently, 14 members of the European Parliament (MEPs) have backed the India-South Africa proposal for TRIPS waiver citing the need to allow the sharing of technology, data, know-how, allowing generics manufacturers to contribute to increasing global availability, including through support for India and South Africa's proposal at the WTO. This waiver once implemented would provide a broad policy space for the WTO members to meet the needs of their constituents during the pandemic crisis.
We hope this article was a useful read.
Please feel free check our services page to find out if we can cater to your requirements. You can also contact us to explore the option of working together.
Best regards – Team InvnTree
This work is licensed under a Creative Commons Attribution-Non Commercial 3.0 Unported License
International civil groups block India’s fight for IPR waiver for Covid Drugs

On October 2, 2020, India and South Africa negotiated with the World Trade Organization (WTO) to renounce any IP rights relating to COVID-19 drugs, vaccines and other related technologies for a restricted period. India and South Africa had suggested that the proposal should be recommended as early as possible to the General Council but the developed countries questioned its relevance and usefulness. As specified in the proposal, the waiver would last for as many years as agreed from the decision of the General Council and would remain intact until widespread vaccination is globally available and fair proportion of the world population has developed immunity towards COVID-19.
In support, South Africa also urged the TRIPS Council that all WTO members should work together to ensure that IP rights do not create barriers to the timely access to affordable medical products indispensable to combat Covid-19. India asserted that, with the development of new diagnostics, vaccines and related technologies for Covid-19, there could be tendencies of arousal of remarkable issues about availability of these developments. Besides, issues regarding making availability of these technologies and developments in sufficient quantities and at economical prices to meet global demand is something which can’t be discarded. Besides, the possibility of concerns regarding critical shortages in medical products can put at grave risk, patients suffering from other communicable and non-communicable diseases. The World Health Organisation (WHO) has supported India and South Africa’s move. In fact, the WHO is in support to ease international & intellectual property agreements on Covid-19 vaccines, treatments & tests in order to make the tools available to all who need them at an affordable cost.
At the same time, International public health advocacy groups are backing India and South Africa’s joint proposal to the WTO to waive off certain provisions of the Trade Related Intellectual Property Rights (TRIPS) for Covid-19 therapeutics, including vaccines. The US, European Union, the UK and Switzerland, and other developed countries have not supported India and South Africa’s proposal. On the other hand, several developing countries, including China, Pakistan, have backed the proposal.
The US has been trying to push India to tighten its IPR regime. The US and the EU have backed India’s proposal on grounds of provision of Section 3(d) of India’s Intellectual property legislation which allows the Indian Patents Controller to deny patents on entities that are not significantly different from their older versions. This prevents pharmaceutical majors from getting fresh patents on medicines with expired patents by making just cosmetic changes in its formation. The US, at the WTO TRIPS council meeting, said that, intellectual property protections needed to be in place to support new research and innovation. According to the US, there is supposed to be no access to the drugs that have not been developed. There should be support in innovation and its essential. The US disassociated from the patent pool call and was in favour of patents for vaccines and medicines and critical role that intellectual property plays.
There is one issue jeopardizing over the Covid-19 pandemic now. If we do not address the intellectual property rights issues in this pandemic, we probably would end up seeing the repetition of AIDS tragedy that occurred in USA. People in US have died for 10 years as patented AIDS medicine was priced at between 10,000 to 15,000 dollars for a year’s supply. Finally, it was Indian patent laws that, till 2004, did not allow patenting of such medicines, helping people to get AIDS medicine at less than a dollar a day. Today, 80% of the AIDS medicines in the world come from India. Hence, it is unlikely that the vaccines for COVID-19 will provide lifetime immunity. Unlike AIDS, where patient numbers were smaller, but COVID-19 is a visible threat for anyone. Any attempt to hold people and governments to ransom on COVID-19 vaccines or medicines, could see the collapse of the entire patent assembly of TRIPS that big pharma has built.
We hope this article was a useful read.
Please feel free check our services page to find out if we can cater to your requirements. You can also contact us to explore the option of working together.
Best regards – Team InvnTree
This work is licensed under a Creative Commons Attribution-Non Commercial 3.0 Unported License
Request for wavier of IP rights relating to COVID-19

India and South Africa in a historical move, on October 2, 2020 have asked the World Trade Organization (WTO) to waive any IP rights (patents, industrial designs, copyrights and trade secrets) relating to COVID-19 drugs, vaccines, diagnostics and other technologies for a limited period. The limited period could extend for a duration of the pandemic or until a fair proportion of the world population develop herd immunity. WTO is an intergovernmental organization with 164 members. The waiver, if granted, would allow the member countries of the WTO to neither grant nor enforce patents or other IP rights relating to COVID-19 for that specified duration. The wavier is not permanent and is valid only for a specific duration. The wavier, if allowed, would be applicable to all the WTO members which include developed, developing and least-developed countries.
The World Health Organization (WHO) has supported India and South Africa’s step. WHO chief Tedros Ghebreyesus in one of his tweets said, “WHO welcomes South Africa’s and India’s recent proposal to WTO to ease international and intellectual property agreements on COVID-19 vaccines, treatments & tests in order to make the tools available to all who need them at an affordable cost”. The Indian domestic pharma industry has backed its government’s move.
The proposal is first submitted to the TRIPS (Trade-Related Aspects of Intellectual Property Rights) Council and then decided at the Ministerial Conference or the General Council. The TRIPS Council is expected to consider the proposal within 90 days and submit a report to the Ministerial Conference for the decision making. The grant of the proposal is based on the general agreement of the WTO members.
India and South Africa’s move gains significance after Gilead, the patent holder of Remdesivir, the only approved drug for the treatment of COVID-19, has its patent licensed in a way that excludes most of the countries and thereby excluding more than half of the world population from benefiting from the lower price generic competition of the drug. The company continues to hold the license after having received over $70 million of public funding to develop the drug. With the exclusive rights, Gilead has priced the Remdesivir drug at a higher price, depriving access to the drug for majority of the population. Therefore, depending on private companies for a solution to the COVID-19 crises is not a solution. The concern extends as vaccines and treatments needed to fight the pandemic could be limited by patents and other IP barriers.
Furthermore, a quantum of patents for COVID-19 vaccines that are in development stages and patents relating to instruments required for treating COVID-19 are being filed. Companies that have received public funding for the development of the vaccine may get exclusive rights for their patents and therefore end up having monopoly over the drug or vaccine which would create barriers to timely access to affordable medical products.
The wavier may allow governments to stand up for public health in solidarity at international level. The wavier may be important for the developing and the least developed countries as these countries may now have an opportunity to procure the basic treatment required for their public health. The wavier would also provide an opportunity for the countries to collaborate in the R&D of COVID-19 vaccine and in scaling up the manufacture and supply of COVID-19 supplies.
We hope this article was a useful read.
Please feel free check our services page to find out if we can cater to your requirements. You can also contact us to explore the option of working together.
Best regards – Team InvnTree
This work is licensed under a Creative Commons Attribution-Non Commercial 3.0 Unported License

 Follow
Follow